Los extractos de semillas de 21 variedades de caraota fueron ensayados para determinar la especificidad hemaglutinante y la actividad mitogénica. Entre las diferentes variedades de caraotas se pueden distinguir cuatro tipos, dos de los cuales son mitógenos. Se aislaron dos fracciones de lectinas (α y b) de cada uno de los cuatro tipos de caraotas. Sus PM fueron estimados por cromatografía de exclusión y los azúcares presentes por cromatografía en papel. La actividad hemaglutinante, la inhibición de la acción hemaglutinante por derivados de azúcares y glucopéptidos, así como la acción mitogénica, se determinaron para las ocho lectinas purificadas y las cuatro preparaciones control. Las fracciones α y b aisladas a partir de dos de los tipos de caraotas mostraron solamente acción mitogénica mínima, mientras que las de los otros dos tipos de caraotas y todas las preparaciones control fueron mitógenos potentes. Todas las preparaciones mitogénicas aglutinaron en altas diluciones tanto a los glóbulos rojos de vaca activados con tripsina como a los de hámster activados con pronasa; sin embargo, algunas preparaciones resultaron inactivas cuando se probaron con los glóbulos rojos humanos o de conejo. An Venez Nutr 2015; 28(1): 74-81.
Palabras clave: Phaseolus vulgaris, leguminosoae, caraotas, lectinas, fitohemaglutininas, glucoproteínas, estimulación de linfocitos.
Extracts of seeds of 21 bean cultlvars were screened for hemagglutinating specifity and for mitogenic activity. Four types could be distinguished in different beans, two of which are mitogens. Two lectin fractions (a and β) were isolated from each of the four bean types. Their MW were estimated by exclusion chromatography and component sugars by paper chromatography. Hemagglutinating activity, inhibition of hemagglutinating action by sugar-derivatives and glyco-peptides as well as mitogenic action were determined for the eight purified lectins and four control preparations. The a and β-fractions isolated from two bean types had only minimal mitogenic action, while those from the other two bean types and all of the control preparations were potent mitogens. All the mitogenic preparations agglutinated trypsin-activated cow red blood cells and pronase-activated hamster red blood cells in high dilutions but some were inactlve when tested with human or rabbit red blood cells. An Venez Nutr 2015; 28(1): 74-81.
Key words: Phaseolus vulgaris, leguminosoae, beans, lectins, phytohemagglutinins, glycoproteins, lymphocyte stimulation.
1 Escuela de Biología, Apartado Postal 10098, Universidad Central Caracas, Venezuela.
* Se reproduce el artículo: Jaffé, W.G., Levy, A., y González, D.I. (1974). lsolation and partial characterization of bean phytohemagglutinins. Phytochemistry. 13: 2685-2693.
The interesting biological effects of phytohemagglutinins or lectins on normal and pathological animal cells have stimulated research in this field in recent years.(1) Considerable efforts have been made by several groups of investigators toward the purification and physical and chemical characterization of the lectins from beans (Phaseolus vulgaris). (2-8)
However, poorly defined seed materials and different purification procedures have been used. The materials thus obtained differed considerably in chemical and in biological properties. Our aim in the present work was to obtain a number of reasonably purified bean lectins from selected seed lots in order to compare their properties. It was hoped that this approach would help to clarify and interpret the contradictory observations reported in the literature.
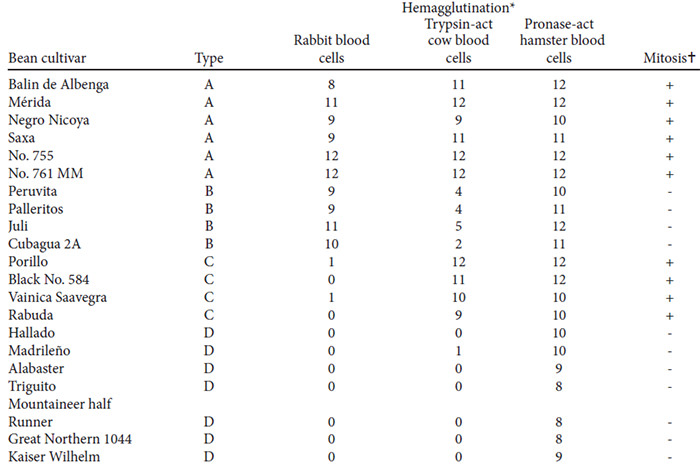
When the hemagglutinating action of extracts of different bean cultivars was measured against different rbc preparations, four specificity types, called A, B, C, and D respectively, were detected (9). The purification of lectins from bean cultivars belonging to these four specificity types will be described in the present paper, as well as some of their physical, chemical, and biological characteristics.
The results of the screening test of 21 bean cultivars for mitogenic activity are presented in Table 1. In all cultures of lymphocytes to which extracts from A or C-type beans had been added, blastic transformation was observed in 30 - 40% and mitotic figures in 45% of the cells. The cultures with added extracts of B or D-type beans never exhibited more than 3%, blastic cells and no mitogenic figures at all.
On chromatography on Biogel P-100 of the fractions obtained by alcohol precipitation of the extracts from the four bean cultivars, hemagglutination activity measured with pronase-activated hamster rbc was confined to the first of the two elution peaks. When the material of these active peaks was submitted to chromatography on DEAE-cellulose, two well separated peaks emerged at about pH 7.9 (α-fraction) and 5.9 (β-fraction). Both contained hemagglutinating proteins. The first peak was small when the extracts form the type-D beans were analyzed, while no important differences were observed between the height of the two peaks with the extracts of the other bean types.
All of the fractions eluted from DEAE-cellulose were subjected to chromatography on Sephadex G-100. In each case this resulted in further separation: a small peak emerged after a much larger main peak. In the present work only the materials of the main peaks were investigated.
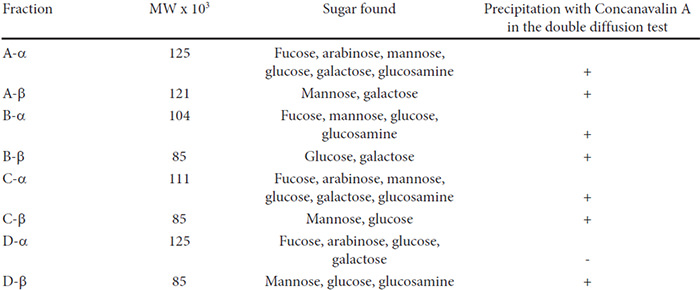
The MWs estimated for the main fractions are presented in Table 2. The electrophoretic mobilities of all purified fractions in polyacrylamide gel slabs at pH 8.9 were very similar. In the microzone electrophoresis, however, the proteins of the β-fractions travelled faster toward the anode than did the α-fractions both at pH 8.8 and 6.25.
At present we have no explanation for these results.
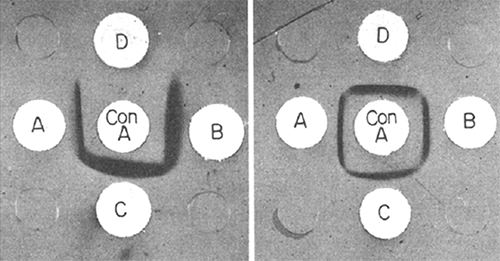
The sugars identified by paper chromatography in the hydrolysates of the lectin fractions are listed in Table 2. The number of different sugars detected varied from two in three of the β-fractions to six in two of the α-fractions. All but one of the lectin fractions were precipitated by Concanavalin A in the double diffusion test (Fig. 1). This precipitation did not occur when the inhibitor α-methyl-D-mannoside was added to the system.
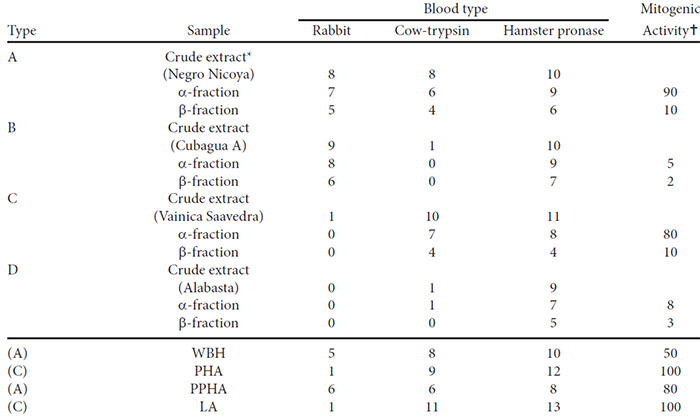
The hemagglutinating action measured with normal rabbit rbc, trypsin-activated cow rbc, and pronase-activated hamster rbc are presented in Table 3. The results show that the specificity differed in the same way as it did in the crude extracts but was identical for the α and β-fractions of any one bean cultivar. All fractions were active against pronase-treated hamster rbc. In all cases, the β-fractions were less active than the α-fractions. Of the control lectins two agglutinated rabbit rbc strongly and two did so only very weakly.
The inhibitory action on hemagglutination of a number of sugar-derivatives and carbohydrate-containing compounds of the lectins used in the present study are included in Table 4. Only the results for pronase-treated hamster rbc are presented because this was the only blood preparation agglutinated by all of the lectins. There were differences between the effects of the lectins from the different bean varieties with respect to their susceptibilites to the inhibitors but the two fractions obtained from each variety behaved in an identical way. Fetuin and N-acetyl-galactosamine inhibited all of the lectins. The A-, B- and C-type bean fractions were also inhibited by pig erythrocyte mucoid and the B-type bean fractions by N-acetyl-glucosamine.
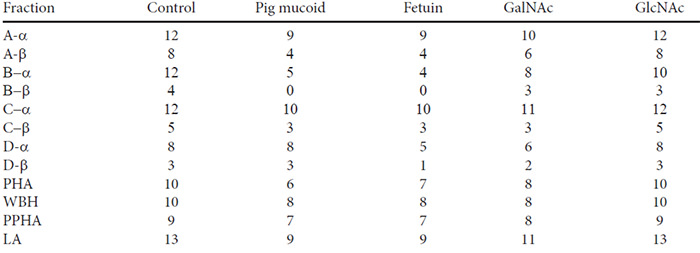
Some results of the tests for mitogenic activity are included in Table 3. It can be seen that the B and D- type lectins showed only minimal mitogenic activity and that the β-fractions were less active than the α-fractions.
The results of a typical adsorption experiment is presented in Table 5. The strong hemagglutinating activity on trypsin-activated cow rbc and the mitogenic activity remained when a mixture of A- and C-type bean extracts was adsorbed with rabbit rbc, while treatment with cow rbc eliminated both activities simultaneously.

The screening test indicates that mitogenic activity is not found in the extracts of all bean varieties. Similar observations had been reported by other authors.10,11 All the mitogenic samples belonged to bean varieties classified as A or C-types according to their rcspective hemagglutinating specificities.
The isolated lectins from these four bean types had the same hemagglutinating specificity as the corresponding crude extracts (Table 3). This means that the action of the crude extracts on different types of rbc and on lymphocytes was not due to the combined activities of different factors, but can be explained by the multiple activity of the lectins isolated from each of these extracts.
The erythro-agglutinating action of all the bean lectins tested was inhibited by fetuin and N-acetyl-D-galactosamime which had previously been shown to possess inhibitory activities under comparable conditions (12,13). Three of the four lectin types were inhibited by pig erythrocyte mucoid, which has been described as a potent inhibitor of kidney bean lectin. (14) In our experiments its activity was rather weak and similar to that of fetuin.
Our results show that the differences in specificity between the four bean lectin types are quantitative rather than qualitative, because a weak agglutinating activity could be detected with high concentrations of lectins of B and D-type beans tested against tripsin-activated cow rbc and of C and D-type lectins tested with rabbit rbc. Also, the “inactive” lectins of the B and D-types had a weak but detectable stimulating action on lymphocytes. The previous observation that bean lectins are absorbed not only by the erythrocytes they agglutinate but to a lesser degree by those cells which are more or less refractory toward their agglutinating action (9) also supports this conclusion.
All fractions were tested repeatedly for mitogenic activity in lymphocyte cultures at concentrations between 75 and 01 mg/mL. Maximal activities were observed at 3-10 mg/mL. The response curves observed by us were less bell-shaped than in the studies of Rigas and Tisdale (15) who obtained maximal response of their bean phytohemagglutinin at a concentration of 5 mg/mL. Leucocytes obtained from different donors responded somewhat differently to the different lectins. The values presented in Table 3 were therefore estimated from several experiments. From these results it is clear that B- and D-type lectins have only minimal mitogenic activity and it also appears that the α-fractions are more active than the β-fractions. Quantitative comparisons of the activities is complicated because of the instability of bean lectins.(4) The WBH used in our experiments had been stored for 4 yr as a lyophilized powder at 4° and our fractions had been kept frozen for 1 yr. Differences in the degree of denaturation, purity, and specific activity could therefore account for the quantitative divergences in biological activities between the different preparations.
Several of the most exhaustively purified bean lectins possess both hemagglutinating and mitogenic activities (2, 4, 8) while some authors claim to have obtained mitogenic preparations without or with only minimal action on red blood cells.(5-7, 16-18) Our results prove that these contradictory observations may be explained if A-type lectins had been obtained by the first group of authors and C-type lectins by the second. Indeed, WBH and PPHA possess the biological properties of A-type lectins while PHA and LA are typical C-type lectins.
Erythrocytes which are not agglutinated by some types of bean lectins may nevertheless bind them, but they are much more active in binding the lectin types which are most efficient in agglutinating that special type of blood cell.9 Therefore, when a mixture of A- and C-type lectins is adsorbed with rabbit blood, the C-type lectin will be concentrated in the supernatant and exhibit mitogenic activity but no significant agglutinating action when tested with rabbit or human rbc (Table 5). The experiments of several investigators (19-22) who claimed to separate erythroagglutinins from mitogens by adsorption of the former on rbc are therefore no proof for the existence of these separate factors as long as they have not been tested with an appropriate rbc preparation.
The carbohydrates found in the eight bean lectins analyzed were all different and were also different from those detected in the bean lectins described by other authors (2, 3, 6, 8). The fact that with one exception the bean lectins are precipitated by Con A indicates that they have some non-reducing D-glucoside or mannoside residues. (23) The 4 control lectins included were likewise precipitated by Con A in the double diffusion test. These precipitations do not occur in the presence of the Con A-inhibitor α-methyl-mannoside. It seems that there are no two bean lectins among all those studied until now which have the same carbohydrate composition.
Our α- and β-fractions had different isoelectric points as revealed by the differences in pH at which they were eluted from DEAE-cellulose and because they differed in their electrophoretic mobilities. Sugar components as well as MWs were also different, but biological activities were qualitatively identical between the lectins obtained from anyone bean sample. The α-fractions had somewhat higher MWs as determined by gel filtration and a greater number of sugar components than the p-fractions. In the former, fucose was a constant component which was not found in the latter. The term “isolectins” has been used for multiple forms of agglutinins isolated from the same seed extracts having identical biological activities and MWs but differing in electrophoretic mobilities and sugar components. (24)
The A-type hemagglutinin specificity is inherited as a single dominant trait as has been found in previous work from this laboratory, (9) but it is found in at least two different isolectins, the α- and β-fractions described in the present paper. These facts can be explained by postulating an active subunit, common to both fractions. The presence of active subunits in bean lectins has been demonstrated by several groups of authors. (1,17, 25, 27)
Yachnin and Svenson have observed five protein bands in the purified active fraction obtained from a crude bean hemagglutinin.(17) They propose a structure composed of four subunits and suggest the existence of two kinds of subunits. By different combinations five different proteins could result in analogy to some isoenzymes like lactic dehydrogenase. One subunit would be responsible for mitogenic and the other for hemagglutinating activity. The presence of both hemagglutinating and mitogenic actions in our α- and β- fractions would mean that both must contain the two types of subunits Nevertheless, according to the data of Table 2 the sugar constituents of the respective α- and β- fractions were different in all cases, contrary to what should have been expected if both consisted of the same kind of subunits in different proportions and if these subunits bear the sugar residues. In order to reconcile our results with the aformentioned hypothesis it must be assumed that the carbohydrate moieties are added to the lectin molecules after these have been assembled from their subunits.
Many commercial bean varieties tested in this laboratory by the single-seed assay proved to consist of mixtures of two or three bean types. When used for fractionation work, these materials may yield several types of lectins. The B-type lectins, when assayed with rabbit or human rbc would appear as erythroagglutinins without significant mitogenic action and the C-type lectins would appear to be mitogens without hemagglutinating action until trypsin-activated cow rbc or pronase-activated hamster rbc are used. On the other hand, the A-type lectins will exhibit both activities when tested with any type of blood. Therefore, trypsin-activated cow rbc should always be used along with the other blood preparations. A bean lectin preparation of known activity should be included in the tests because some cow blood samples will not produce a normal reaction, (9) a fact which is probably related to the different blood groups of cattle. (28)
It is noteworthy that the bean types most active in mitotic stimulation are also the most toxic. (29) It seems possible that both activities are somehow related. Hopefully, further in sight into the structural details of these closely related lectins may be useful for exploring the features required for the mitogenic activity and toxicity.
Preliminary screening test. Extracts from seeds of 21 genetically pure lines of beans grown in the experimental field of this Departament were tested for hemagglutinating action. Single seeds were ground in a mortar and weighed portions extracted with 10 parts of a 1% NaCl soln for 2 hr. Suspensions of washed red blood cells (rbc) were added to aliquots of serial dilutions of these extracts. Native cow rbc are not agglutinated by bean phytohemagglutinins (9), In order to make them susceptible to hemagglutination they were trated for 30 min with a 0.1% soln of crystallized trypsin in physiological saline, washed 3 x, and resuspended. Hamster rbc were activated by treatment with 0.1- pronase for 1 hr. Rabit rbc were used on the native form. Hemagglutination tests were performed using the microtitration kit (Cooke Engineering Comp., Alexandria, Virginia). Because some cow blood samples are refractory to hemagglutination by bean lectins even after trypsin-activation a control lectin preparation of known activity was included in every test.
Mitogenic activity. Crude extracts obtained from single seeds of these 21 bean cultivars were tested in duplicate cultures of human lymphocytes from 2 different donors by the microscopic observation technique of Favier et al. (30)
Purification of the lectins. Seed samples of each one of the 4 specificity types were selected and uniformity of the lots was verified by testing at least 50 single seeds with the 3 blood cell preparations described above. The selected bean cvs were: Negro Nicoya, black, type A- Cubagua, subline, black, type B; Vainica Saavedra, black, type C; Alabaster, white, type D. Ground seeds (20 g) of each of the 4 selected bean samples were suspended in 200 mL of H2O, adjusted to pH 4 and stirred for
24 hr at 4°, following centrifugation the supernatants were heated to 80° for 10 min, neutralized and clarified by centrifugation at 2°. EtOH of the same temp. was slowly added with mechanical stirring. The ppts which were formed by addition of EtOH in the range of 33-66% were dissolved in Tris-HCl 0.01 M, pH 7.6 and passed through a 250 mL column of Biogel P-100. The fractions contained in the first elution peaks were concn under red.pres. The pH was adjusted to 8 and the material was passed through a DEAE-cellulose column stabilized at
pH 8 with 0.05 M Tris-HCl buffer. The column was washed with Tris-succinate buffer of a pH gradient varying from 8.38. The eluates of the two main peaks which emerged at pH 7. 9 and 5. 9 were dialysed, concn and passed through a column of Sephadex G-100 which had been calibrated with the following proteins: pepsin, ovalbumin, trypsin, WBH and hemoglobin. A calibration curve in which the MWs were plotted against the respective elution vol was constructed and the MWs of the bean fractions were estimated from their corresponding elution vols. The eluates of the peaks were concn and frozen.
Incorporation of the thymidine [3H] into DNA by the isolated lectins was tested as follows to 2 ml duplicate cultures of about 14 x 106 human lymphocytes purified by glass bead chromatography (31) labeled thymidine (1 µCi/culture) was added after 48 hr of cultivation, and the latter was allowed to continue for another 20 hr. The thymidine incorporation into the DNA was measured in a liquid scintillation counter. (32) Each fraction was tested 3-5 times at levels between 75 and 0.1 µg/mL. For the agglutination-inhibition tests the lectins were titrated in the ‘Microtiter” equipment using a soln of the inhibitors in physiological saline as the diluent. The following inhibitors were used N-acetyl-D-glucosamine (Serva). N-acetyl-D-galactosamine (Sigma), fetuin (Calbiochem), and pig erythrocyte mucoid. (14)
Double diffusion tests. Performed in 1.25% agar plates using 0.5% solns of the bean lectins and in the center well a 1% soln of Con A. Sugar analysis. After hydrolysing 5 mg portions of the lyophylized fractions for 2 hr at 100° with 2N H2S04, sugars were determined by ascending paper chromatography. They were revealed with AgNO3 in acetone-H2O or with aniline-phthalate and the amino-sugars by the Morgan-Elson technique as described earlier. Pure sugars were chromatographed simultaneously with the experimental samples. Crude bean extracts and washed and packed red blood cells were mixed for the adsorption experiments in the proportion of 2:1. After 30 min of agitation the mixture was centrifuged and the procedure repeated. Hemagglutinating and mitogenic activities were determined in the supernatants after each adsorption.
Electrophoresis. The protein fractions were subjected to microzone electrophoresis in phosphate buffer of pH 7.9 and 6.2 and to electrophoresis on vertical polyacrylamide gel slabs. The discontinuous technique was used, the running gel being 10% Cyanogum-41 in 0.50 M Tris-HCl buffer, pH 8.9 and the spacer gel 5% Cyanogum-41in 0.10 M Tris-HCl buffer pH 6.7. Both buffers contained 0.24% TEMED and 0.0024% Tween 80. Electrode buffer was Tris-glycine 0.05 M, pH 8.4. For comparison samples of commercial bean hemagglutinin (PHA) (Wellcome Lab lot No. K 4403), wax bean hemagglutinin (WBH),(2) kidney bean leucoagglutinin (LA)(7) and phytohemagglutinin (PPHA)(8) were included in the tests for biological activity.
We are indebted to Dr I. Posner for performing the analytical polyacrylamide electrophoresis and for useful suggestions in the preparation of this manuscript, to Mr A. Callejas for some of the hemagglutination tests, to Mrs V. Gómez for the mitosis tests, to Mrs M. Jaffé for some sugar analysis. We thank Dr I. E. Liener for a gift of WBH. Dr D. A. Rigas for a gift of PPHA, Dr T. Weber for a gift of kidney bean leucoagglutanin (LA), Dr G. Uhlenbruck for a gift of pig mucoid. The financial support of the Consejo del Desarrollo Científico y Humanístico of the Central University of Venezuela and the CONICIT is gratefully acknowledged.